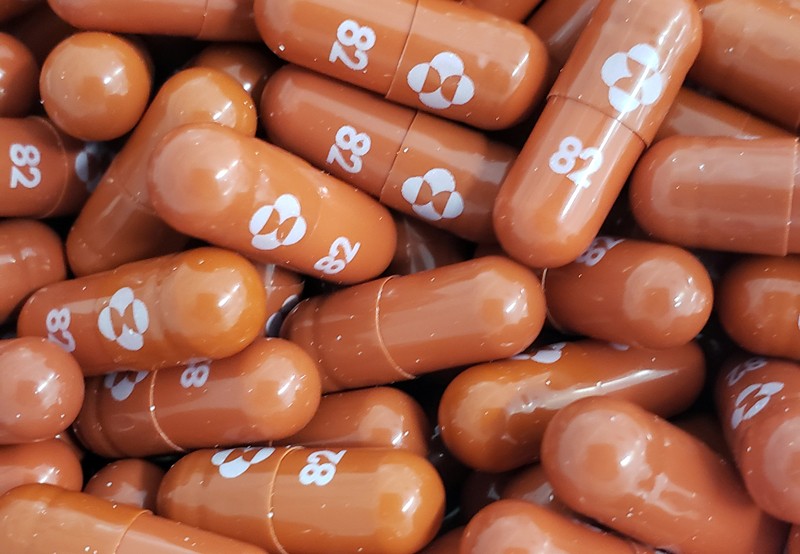
The pharmaceutical firm Merck announced last week that an antiviral pill it’s developing can cut hospitalizations and deaths among people with COVID-19 by half. The results haven’t yet been peer reviewed. But if the drug candidate, molnupiravir, is authorized by regulators, it would be the first oral antiviral treatment for COVID-19. By contrast, the other currently authorized drugs must be delivered intravenously or injected.
A pill could make treating patients earlier on in their infection much easier — and more effective. It could also keep hospitals from overflowing, especially in places where vaccination rates are still low, such as many low- and lower-middle-income countries. Molnupiravir was so effective in a phase 3 trial involving COVID-19-positive people at risk of severe illness that clinicians halted enrolment early.
But whether this clinical-trial success story will translate into a global game-changer in the fight against the pandemic isn’t yet clear. This week, two Indian drugmakers testing molnupiravir in people with moderate illness due to COVID-19 sought to end their trials because they saw no “significant efficacy” for the experimental drug. Also, Merck’s findings, which apply to people with mild-to-moderate cases of COVID-19, were disclosed only in a press release — and have yet to be pored over by scientists and submitted to regulators for approval. And even if lower-income countries can afford the medicine, they might not have the diagnostic capacity to treat patients with molnupiravir early in the course of their illness, when treatment could make a difference.
Hit early, hit hard
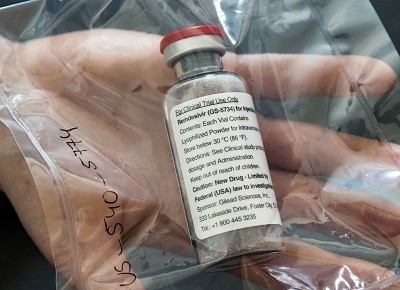
The other therapies on offer against COVID-19, Gilead Science’s antiviral remdesivir and a monoclonal antibody cocktail from biotech firm Regeneron, must be administered intravenously or by injection. That makes it difficult for people to access the therapies before they are sick enough to land in hospital. And remdesivir is approved only for those who are already hospitalized with COVID-19.
Yet it’s better to “hit early, hit hard” with antivirals, says Richard Plemper, a virologist at Georgia State University in Atlanta. The sicker the patient, the less effective the drugs are at treating the illness. A COVID-19 pill, which simply requires a prescription and a trip to the pharmacy once symptoms appear, would make early treatment much easier.
COVID-19 is not the first disease caused by a coronavirus to seriously impact humans. But the 2002–04 severe acute respiratory syndrome (SARS) epidemic fizzled out quickly, and the Middle East respiratory syndrome (MERS) outbreak in 2012 never became widespread — meaning that drugmakers had little incentive to develop antivirals against these diseases.
So when the first cases of COVID-19 emerged in late 2019, “there wasn’t a portfolio of antivirals waiting”, says Saye Khoo, an infectious-disease physician at the University of Liverpool, UK, who has led a clinical trial of molnupiravir.
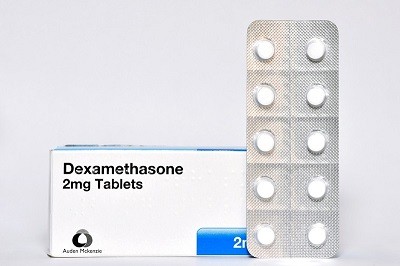
Initial efforts to find treatments focused on drugs already approved by regulators, and yielded only one winner: dexamethasone, a steroid aimed at dampening an overblown inflammatory response in the sickest people. The FDA has not authorized the drug for this purpose, but it's widely used to treat the sickest people.
But even as researchers scrambled to test approved drugs, pharmaceutical companies and biotechnology firms were scouring their libraries for any compounds with known antiviral activity that might stop the SARS-CoV-2 coronavirus. These broad-acting antivirals weren’t designed specifically to target SARS-CoV-2, but it seemed mechanistically feasible that they could. Unlike with many of the drugs tested early in the pandemic, “there’s a scientific rationale. You understand how they’re working”, says Jay Luly, chief executive of Enanta Pharmaceuticals, a company in Watertown, Massachusetts, that is developing its own COVID-19 antiviral.
So far, Gilead’s remdesivir is the only such drug that has received approval from the US Food and Drug Administration. When used in a hospital setting, its effect is modest. In a phase 3 trial, researchers found that it shortened recovery time by a median of 5 days1. Merck hopes molnupiravir will be next to receive authorization.
Hot pursuit
Molnupiravir began as a possible therapy for Venezuelan equine encephalitis virus at Emory University’s non-profit company DRIVE (Drug Innovation Ventures at Emory) in Atlanta. But in 2015, DRIVE’s chief executive George Painter offered it to a collaborator, virologist Mark Denison at Vanderbilt University in Nashville, Tennessee, to test against coronaviruses. “I was pretty blown away by it,” Denison remembers. He found that it worked against multiple coronaviruses: MERS and mouse hepatitis virus2.
Painter also recruited his collaborator Plemper to test the drug against influenza and respiratory syncytial virus. After the pandemic hit, however, plans changed. DRIVE licensed the compound to Ridgeback Biotherapeutics in Miami, Florida. Plemper, too, pivoted to coronaviruses, and tested the compound in ferrets. It silenced the virus’s ability to replicate, he says, but it also suppressed the virus’s transmission from infected ferrets to uninfected ones3. Merck’s data hint that might also be true in humans: molnupiravir appeared to shorten the duration of SARS-CoV-2’s infectivity in trial participants with the virus.
Molnupiravir, like remdesivir, is a nucleoside analogue, which means it mimics some of the building blocks of RNA. But the compounds work in entirely different ways. When SARS-CoV-2 enters a cell, the virus needs to duplicate its RNA genome to form new viruses. Remdesivir is a ‘chain terminator’. It stops the enzyme that builds these RNA ‘chains’ from adding further links. Molnupiravir, on the other hand, gets incorporated into burgeoning RNA strands and, once inside, wreaks havoc. The compound can shift its configuration, sometimes mimicking the nucleoside cytidine and sometimes mimicking uridine. Those RNA strands become faulty blueprints for the next round of viral genomes. Anywhere the compound gets inserted and that conformational shift happens, a point mutation occurs, Plemper says. When enough mutations accumulate, the viral population collapses. “That is what we term lethal mutagenesis,” he adds. “The virus essentially mutates itself to death.” And because the mutations accumulate randomly, it’s difficult for viruses to evolve resistance to molnupiravir — another plus for the compound.
But the compound’s mutagenic potential in human cells — the possibility that it could incorporate itself into DNA — does raise safety concerns, some researchers say. Merck hasn’t released any detailed safety data yet, but “we’re very comfortable that the drug will be safe if used as intended”, said Daria Hazuda, Merck’s vice-president of infectious-disease discovery and chief science officer, at a press briefing last Friday.
Waiting in the wings
Other antivirals are in the works. Gilead Sciences is developing a pill version of remdesivir. And Denison suspects that if the antiviral were given to people as early as molnupiravir is — when symptoms have only just appeared and viral loads are high — it would be similarly effective. In a study presented at IDWeek, a virtual meeting of infectious-disease specialists and epidemiologists held earlier this month, researchers reported results of administering infusions of remdesivir to people in the early stages of COVID-19 every day for three days. The number of participants in the study was small, but remdesivir appeared to reduce hospitalizations by 87% in people at high risk of developing COVID-19.
Biotech firm Atea Pharmaceuticals in Boston, Massachusetts, also has an antiviral in the works. It was testing a nucleoside analogue against hepatitis C in a clinical study when SARS-CoV-2 emerged. The pandemic paused the trial, so Atea decided to switch its focus to COVID-19. Now it has partnered with Roche in Basel, Switzerland, to develop its compound.
Pfizer, based in New York City, had a bit of a head start too. The company had been developing antivirals against SARS since the early 2000s, but shelved them when the outbreak ebbed. When the COVID-19 pandemic began, “they just blew the dust off”, Luly says. Researchers are currently testing a pill form of a compound that has a mechanism of action similar to those original versions. It is in phase 2/3 trials for treating people who are newly infected.
Global access
An effective oral antiviral would be an incredible asset in the fight against COVID-19, but it’s not yet clear whether molnupiravir will be accessible to all. “Are we going to be in a situation where the price is reasonable in low- and middle-income countries?” asks Rachel Cohen, the North American executive director at the Drugs for Neglected Diseases initiative.
The United States has agreed to purchase 1.7 million courses of molnupiravir for US$1.2 billion, which works out to about $700 per 5-day course. That’s far less than the price of remdesivir or monoclonal antibodies, but still too costly for much of the world. Merck, which is co-developing the compound with Ridgeback, has struck licensing agreements with five Indian manufacturers of generic drugs. Those deals allow the manufacturers to set their own price in India and 100 other low- and lower-middle-income countries.
But even if poorer countries can afford the drug, they might not have the diagnostic capacity to use it properly. If molnupiravir needs to be given in the first five days after symptom onset, “that requires that we are able to actually rapidly diagnose people”, Cohen says. For many developing countries — and even some wealthy ones — “that is actually a huge challenge”.
"how" - Google News
October 09, 2021 at 05:54AM
https://ift.tt/3AqUgVk
How antiviral pill molnupiravir shot ahead in the COVID drug hunt - Nature.com
"how" - Google News
https://ift.tt/2MfXd3I
Bagikan Berita Ini















0 Response to "How antiviral pill molnupiravir shot ahead in the COVID drug hunt - Nature.com"
Post a Comment